Baculovirus–insect Cell Expression System
The baculovirus–insect cell system is a eukaryotic expression system, in which the translational and post-translational modification are similar to those of mammalian expression systems. This system uses baculovirus as a vector to introduce foreign genes and infect insect cells to express proteins. It is widely used for expression proteins (such as GPCRs, ion channels, kinases, and other toxic proteins) that may negatively impact mammalian cells.
Advantages of the baculovirus–insect cell system
1.The system has a complete protein processing and modification pathways, such as glycosylation, acetylation, and phosphorylation;
2.The system enables correct folding and disulfide bond formation of a protein. Therefore, the system is widely used for making proteins with complicated structures;
3.The system has the protein sorting capacity, such as directing a nuclear protein into the nucleus, translocating a membrane protein to the plasma or organelle membrane, and secreting a protein outside the cell;
4.Insect cells can be cultured in suspension, thereby allowing massive production of a recombinant protein. The recombinant protein is of high expression level, probably over 50% of the total protein;
5.The system can express proteins in large size (~200kD) without affecting cell proliferation;
6.The system has the ability to simultaneously express multiple proteins within a single cell;
7.The system has a wide application and can be used to express proteins from a variety of sources, including viruses, bacteria, fungi, plants, and animals. The system can also process mRNA consisting of introns;
8.The system is safe to vertebrates. Baculovirus is a class of insect viruses that has a restricted host range. It is not pathogenic to vertebrates and plants. The recombinant baculovirus is vulnerable in nature, as the virus is loss of the protection from its polyhedral capsid.
The classification of baculovirus-insect cell systems
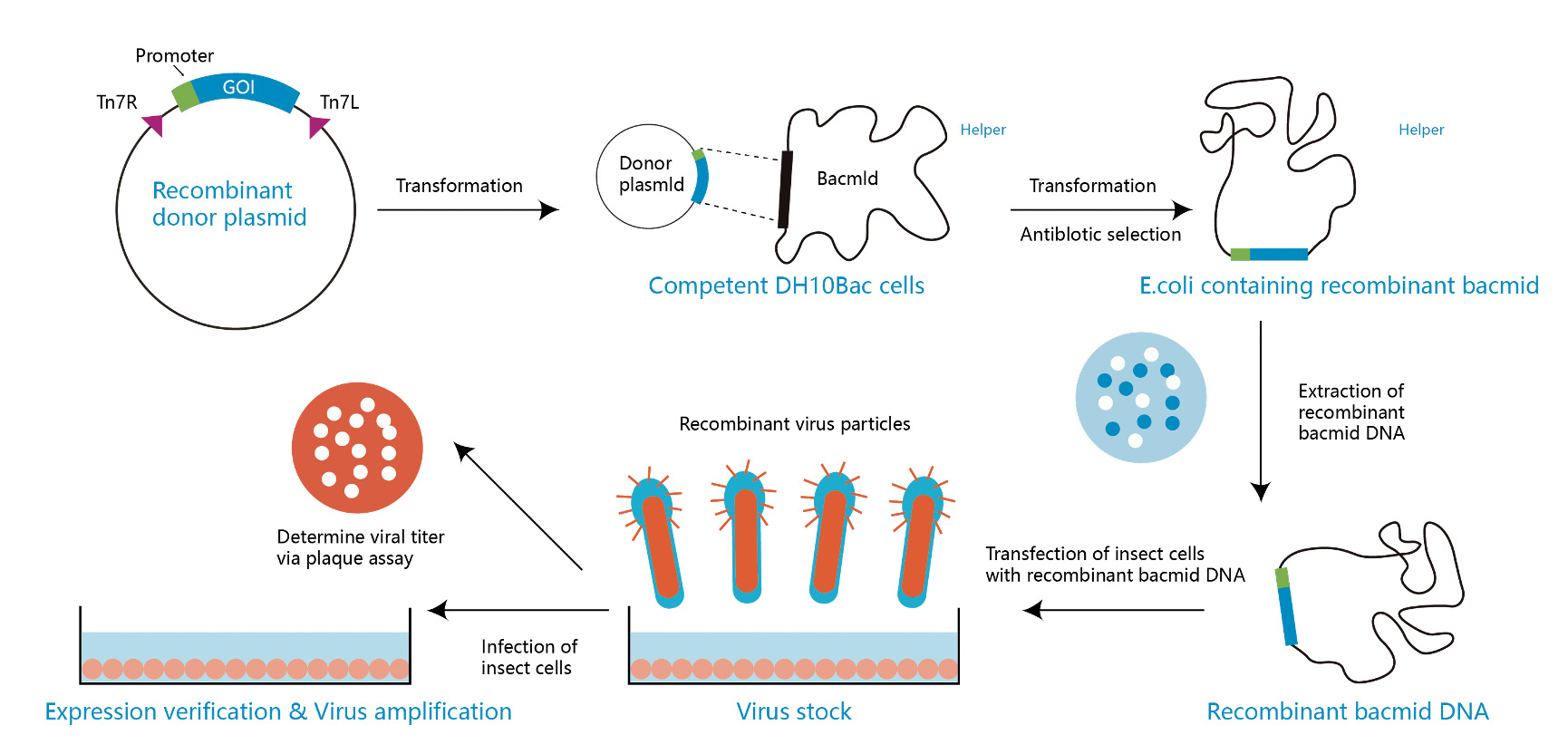
Bac-to-Bac expression system
The system can effectively generate recombinant baculoviruses for protein expression. The recombinant baculoviruses are generated in E.coli through site-specific transposition, rather than homologous recombination in insect cells. First, the pFastBac vector is recombined with a defective parent baculovirus plasmid in DH10Bac E. coli cells to form a large plasmid (termed bacmid). Then, the recombinant baculovirus particles are generated by transfecting insect cells with bacmids. This baculovirus uses the p10 and polyhedrin promoter to drive exogeneous protein expression in various insect cell lines, such as Sf9, Sf21, and HighFive cells.
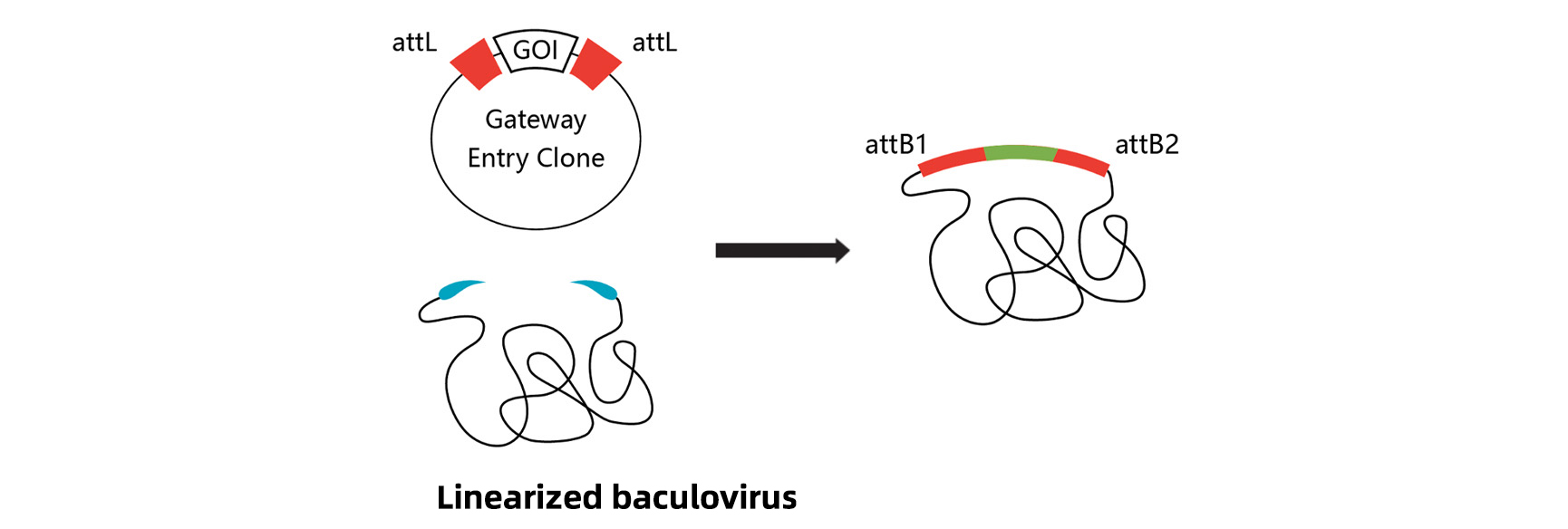
BaculoDirect Expression System
Linearized baculovirus DNA contains attR sites at both ends, with the HSV1tk gene inserted between the two attR sites. The gene of interest is cloned into an entry vector using Gateway technology. Then the linearized baculovirus DNA and entry vector are both transfered into insect cells to make recombinant viruses through LR reaction. The viruses containing the gene of interest are obtained by negative selection with ganciclovir.
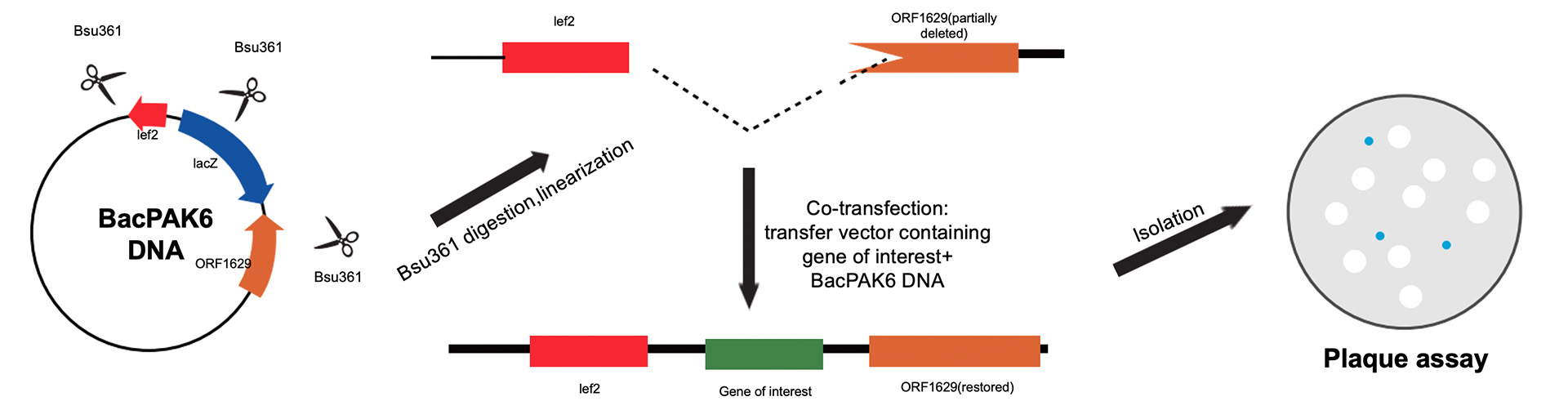
BaculoGOLD and BacPAK6 expression systems
Both systems use Bsu36I to linearize the baculovirus DNA, in which a deletion in the ORF1629 region of the nucleocapsid gene disables the replication capability of the gene in insect cells. However, the active virus that regains missing region of the nucleocapsid gene can be generated in the insect cells where the linearized virus DNA and transfer vector containing exogenous gene take a recombinant reaction.
FlashBAC system
Unlike BacPAK6 and BaculoGOLD, the missing fragment of the replication essential gene (ORF1629 of the nuclear-capsid-protein encoding gene) of AcNPV DNA is replaced by a synthesized fragment in the polyhedrin coding region. The modified circular baculovirus can no longer replicate in insect cells. However, the missing fragment of replication essential gene of the virus is regained through homologous recombination when insect cells are co-transfected by the transfer vector containing a target gene and circular baculovirus. Only viruses that successively undergo homologous recombination can replicate in insect cells, thus achieving one-step production of recombinant baculovirus in insect cells without need of any screening.

AlpHelix offers services for baculovirus generation and insect cell-based protein expression
With rich experience, optimized expression vectors, and high titer virus packaging technology, AlpHelix provides unparalleled one-stop services from baculovirus preparation to protein expression using insect cell systems. The services cover multi-pass membrane proteins (GPCR, ion channels and solute carriers), tyrosine kinases, and other challenging (e.g. high molecular weight) soluble proteins.
| 1-2weeks | 1-2weeks | 2-3weeks | 2-3weeks | 3-5weeks |
| Gene synthesis and codon optimization (optional) | Generation of expression constructs | Expression test and solubility analysis | Pilot expression and purification | Large-scale expression and purification (optional) |
| Customer provides protein sequence information or plasmid information | · Cloning genes into expression vectors · vector sequencing · vector preparation Deliverables · Sequencing report (if requested by the customer) |
· Virus packaging · Virus amplification |
· Transfecting insect cells with baculoviruses · Small-scale expression test, optimizing protocols for protein purification in their soluble forms · QC analysis (SDS-PAGE, UV, etc.) Deliverables · 0.1-0.5 mg purified protein (if protein’s expression level is reasonable) · Certificate of Analysis (COA) |
· 100mg and above · QC analysis Deliverables · Purified protein · Certificate of Analysis(COA) |
For more information about our technology and services, please contact us.
Contact Us