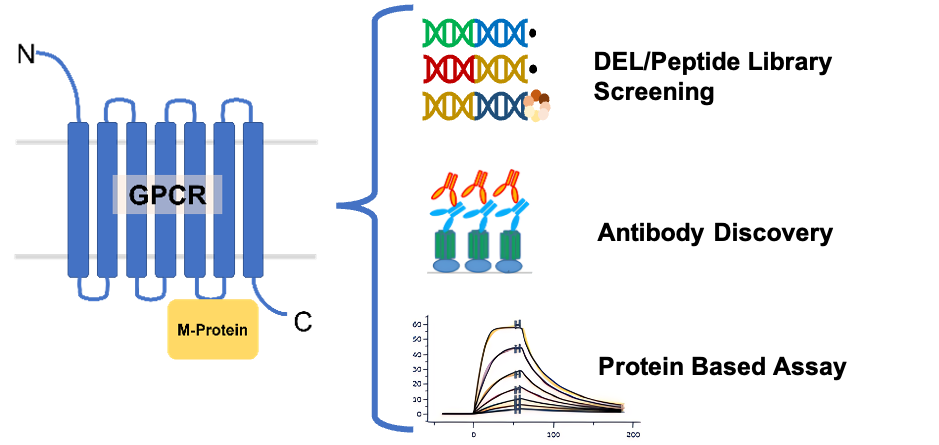
GPCR Preparation
Using its proprietary MegaR technology platform, Alphelix offers the industry's leading GPCR preparation services.
Technical Advantages:
1. Proteins are stable without ligand binding;
2. Possess natural conformation and ligand binding capability;
3. Suitable for DEL screening, AS-MS screening, antibody discovery/screening, and ligand affinity measurement;
4. Protein molecular weight increased to over 100 kD, with structural rigidity, suitable for low-temperature electron microscopy studies;
5. Short preparation cycles for both small-scale and large-scale production;
Services and Duration
| Item | Service Details | Time(weeks) |
| GPCR Protein Preparation | Plasmid design and construction | 1-2 |
| Small-scale expression | 2 | |
| Large-scale expression | 2 |
