X-ray Crystallographic Structure Determination Based on Lipidic Cubic Phase
The lipidic cubic phase (LCP) technique has been widely used for the crystallization of membrane proteins
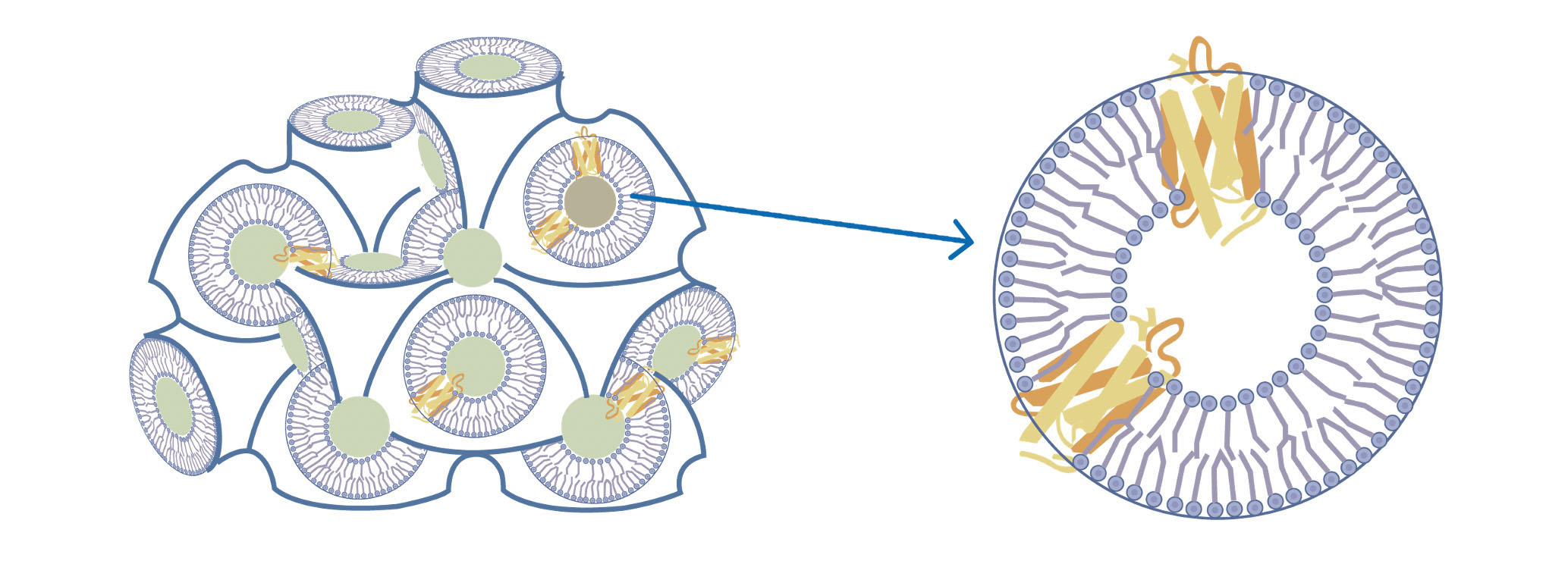
The lipidic cubic phase (LCP) has been widely used as a technique for crystallizing membrane proteins. LCP consists of a single lipid bilayer that follows an infinite periodic minimal surface dividing the space into two non-intersecting networks of water channels. The lipid bilayer helps membrane proteins maintain their native conformations. LCP has successfully aided in the crystallization and drug development of many GPCRs. Scientists at AlpHelix have developed for the first time in the world a method of introducing heavy atoms into LCP crystals. Consequently, membrane proteins with novel architectures can be resolved once these proteins are crystallized by the LCP technique.
Membrane proteins embedded in the lipid bilayer of LCP
Business cases of AlpHelix
By combining the sitting-drop vapor diffusion method and the lipidic cubic phase technique, AlpHelix can introduce heavy atoms into protein crystals grown in LCP. This, together with mini-beam X-ray crystallography, paves the way for resolving novel structures of membrane proteins
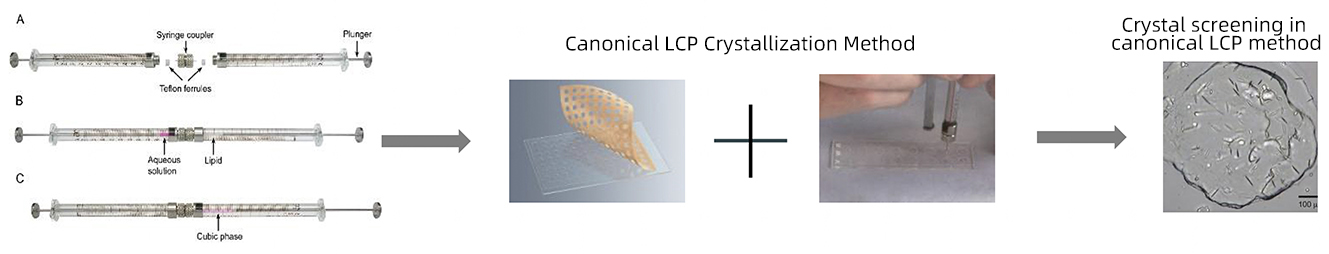
Disadvantages of the traditional LCP method: unable to introduce heavy atoms to the protein crystals; difficult to pick up LCP crystals; hard to determine the protein structure of a novel fold
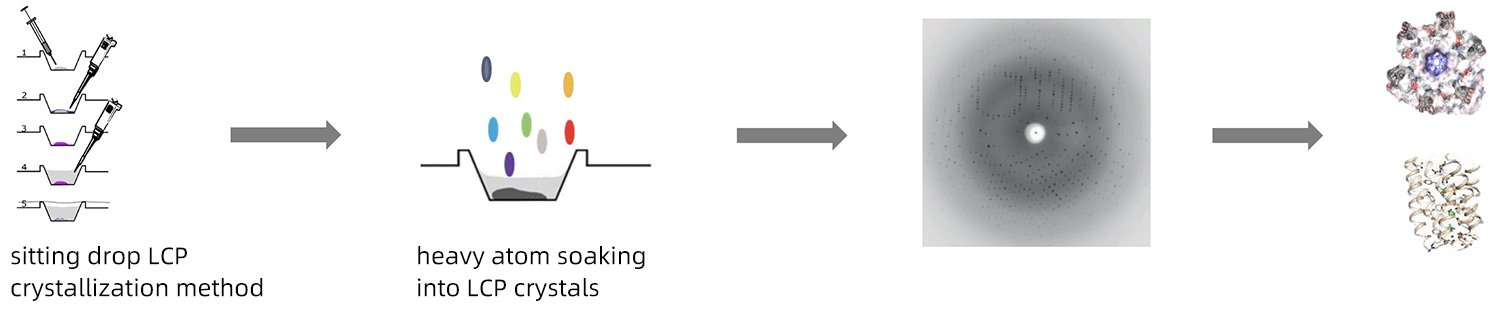
Advantages of sitting-drop vapor diffusion LCP crystallization: convenient in introducing heavy atoms into protein crystals; easy to pick up LCP crystals; ab initio structure determination is easy
The three-dimensional structure of a sodium-calcium exchanger at the 1.9Å resolution. The sodium-calcium exchanger (Samarium-soaked) was crystallized in LCP + X-ray mini beam crystallography
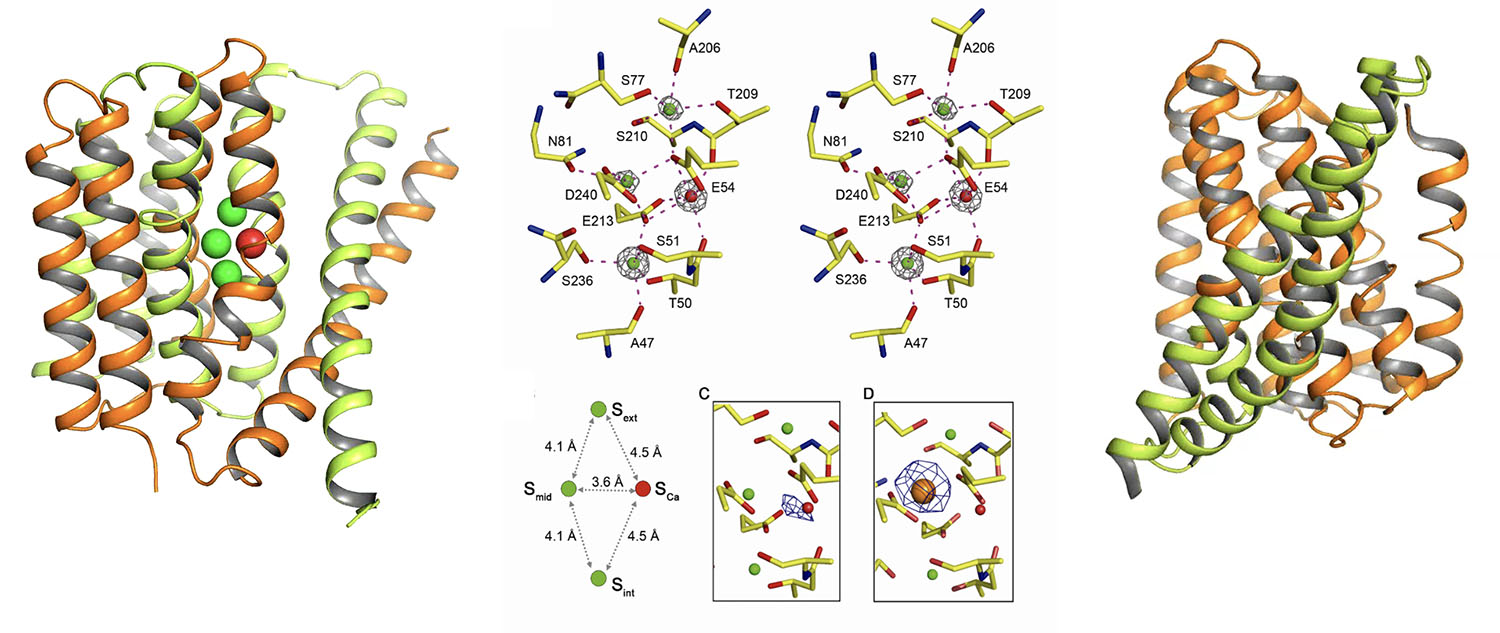
Sodium-calcium exchanger in outward-facing conformation, with green spheres representing sodium ions and red spheres representing calcium ions
Details of coordination for the sodium and calcium ions at the ligand binding site
Model of the outward-facing to inward-facing conformational transition of the sodium-calcium exchanger
Science (2012) 335, 686-690
A series of three-dimensional structures of the sodium-calcium exchanger with resolutions of 2.1 to 2.8 Å. All crystals were grown using the LCP technology
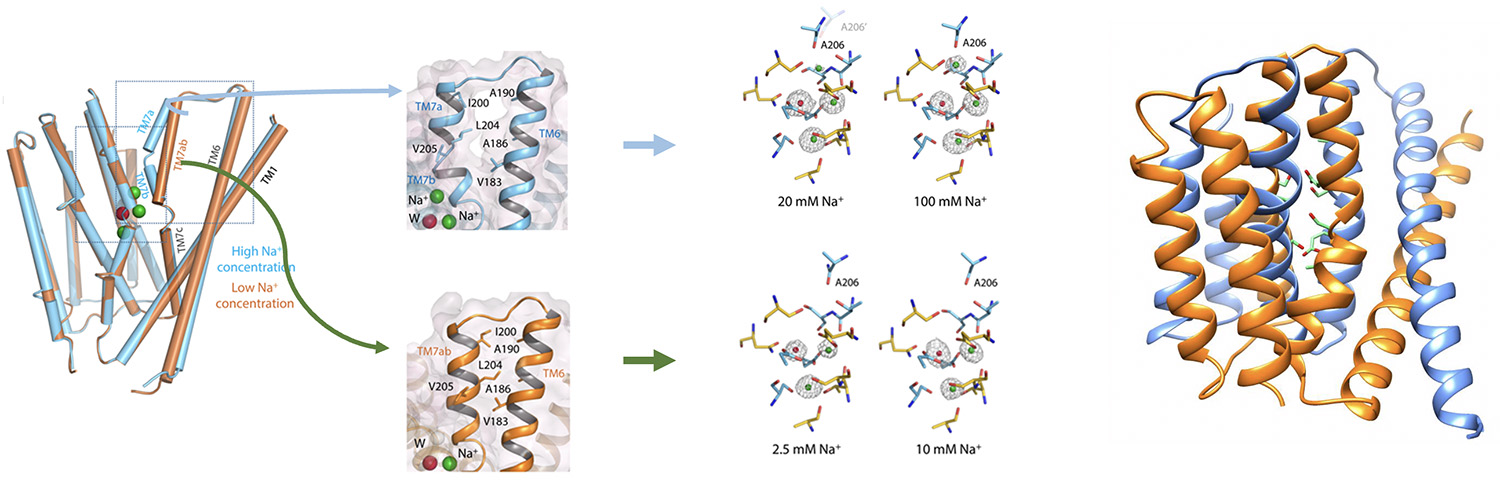
The occupancy of sodium ions in the ligand binding site of the sodium-calcium exchanger
Conformational changes of the TM7 for the sodium-calcium exchanger
Nat. Struct. Mol. Biol. (2016) 23(6), 590-599
Crystal structure of a transmembrane ion channel resolved at 1.9Å resolution using micro-focused X-ray diffraction from heavy-atom-labeled lipidic cubic phase crystals.
LCP crystal photo
Cell Res. (2018) 6, 644-654
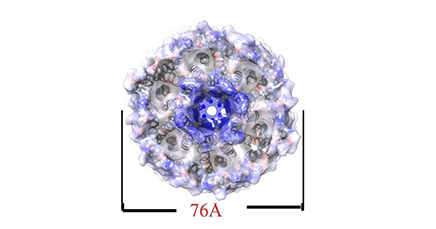
Hexameric structure of the acetate transporter protein SatP_Ck
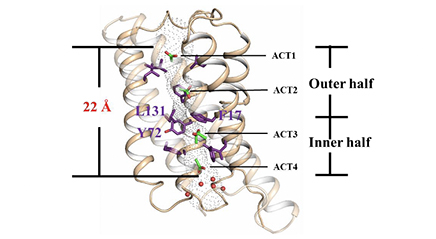
Monomeric structure of SatP_Ck
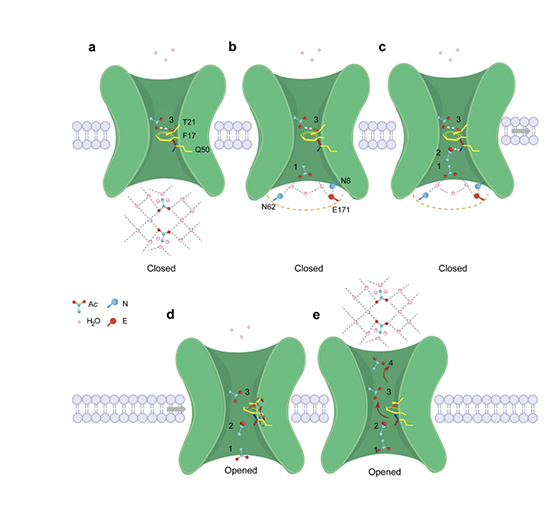
The permeation mechanism of acetic acid in SatP_Ck
1.LCP MAG8.7 crystallography+ LCP heavy-atom soaking method to obtain experimental phase angles
2.The first high-resolution structure of an organic anion-channel protein complex
3.Reveals a unique substrate transport mechanism for organic anions that differs from traditional inorganic ion channels (Na+ channels, Ca2+ channels, K+ channels)
